Avelumab Merkel Cell Carcinoma
Merkel cell carcinoma MCC is a rare and aggressive skin cancer that can metastasize rapidly. Tumor cells make PD-L1 to help them evade being detected by the immune system.
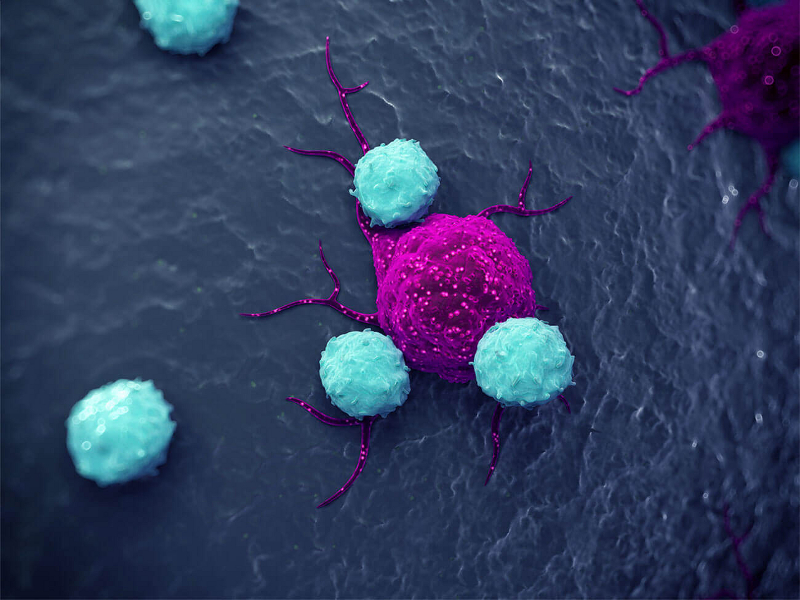
Bavencio Avelumab For The Treatment Of Metastatic Merkel Cell Carcinoma Clinical Trials Arena
Grade 3 treatment-related adverse events occurred in 8 of 39 patients 205 with no treatment-related deaths.
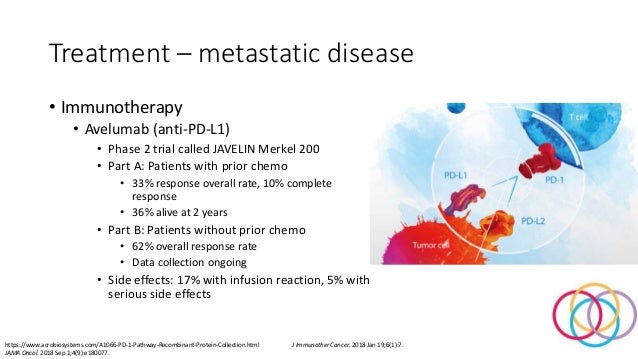
Avelumab merkel cell carcinoma. Meaning First-line avelumab monotherapy in patients with metastatic Merkel cell carcinoma was associated with early responses high. With Avelumab towards reducing the risk of Merkel cell Merkel cell Merkel cells are found in the lower part of the epidermis. In Europe approved systemic therapies are limited to the PD-L1 inhibitor avelumab.
Up to 10 cash back Avelumab Bavencio is a fully human IgG1 monoclonal antibody that is directed against programmed cell death ligand 1 PD-L1. In a clinical trial a new immunotherapy drug called avelumab has shown promise in patients with metastatic Merkel cell carcinoma a rare aggressive skin. Food and Drug Administration today granted accelerated approval to Bavencio avelumab for the treatment of adults and pediatric patients 12 years and older with metastatic Merkel cell.
Abstract Background Merkel cell carcinoma MCC is associated with high recurrence rates and poor survival when metastatic disease is present. Avelumab was associated with durable responses most of which are still ongoing and was well tolerated. Avelumab functions as an immune checkpoint inhibitor and has recently been approved in the USA the EU and Japan for the treatment of metastatic Merkel cell carcinoma MCC.
Avelumab Bavencio an antiprogrammed death ligand-1 anti-PD-L1 immunoglobulin G1 IgG1 monoclonal antibody was approved by the US Food and Drug Administration FDA in. Prior to the approval of avelumab there were no FDA approved treatments for patients with metastatic disease. The FDA has granted an accelerated approval to the PD-L1 inhibitor avelumab Bavencio for the treatment of adults and pediatric patients 12 years and older with metastatic Merkel cell carcinoma.
It is thus the first therapeutic agent specifically. The patient experts explained that Merkel cell carcinoma often progresses rapidly and can. A search of the literature did not find strong evidence to support the use of avelumab for the treatment of metastatic Merkel cell carcinoma MCC.
Although the exact function of Merkel cells. Furthermore there is limited published literature regarding treatment of these patients and no specific regimens are currently recommended by guidelines. Avelumab is an immunotherapy drug that works by blocking a protein in tumor cells called PD-L1.
The expert reference panel supported publication of the protocol on the basis of the information summarised below. Hence avelumab represents a new. This may affect decisions on using avelumab.
The immune checkpoint inhibitor avelumab has shown high response rates RRs and durable responses in patients with. People with metastatic Merkel cell carcinoma would welcome avelumab as a treatment option. In patients with metastatic MCC mMCC brain metastases are uncommon but are associated with poor prognosis.
The ADAM Adjuvant Avelumab in Merkel trial aims to study the effectiveness of immunotherapy immunotherapy A therapy that improves the function of the cells that recognize and destroy foreign objects in your body such as a virus bacteria or cancer. Merkel cell carcinoma MCC is a rare and aggressive neuroendocrine cutaneous malignancy with poor prognosis. By blocking this protein avelumab may improve the ability of the immune system to find and destroy a cancer.
There is an unmet clinical need for people with the disease. Avelumab is approved for treating metastatic Merkel cell cancer. Evidence-based recommendations on avelumab Bavencio for metastatic Merkel cell carcinoma in adults.
Avelumab functions as an immune checkpoint inhibitor and has recently been approved in the USA the EU and Japan for the treatment of metastatic Merkel cell carcinoma MCC. For avelumab-refractory patients efficient and safe treatment options are lacking. Merkel cell carcinoma is a rare aggressive neuroendocrine tumor of the skin.
It is thus the first therapeutic agent specifically approved for use in this indication and is. A table of NHS England interim treatment regimens gives possible alternative treatment options for use during the COVID-19 pandemic to reduce infection risk. Merkel cell carcinoma is a rare and aggressive cancer with limited treatment options.
Merkel cell carcinoma.
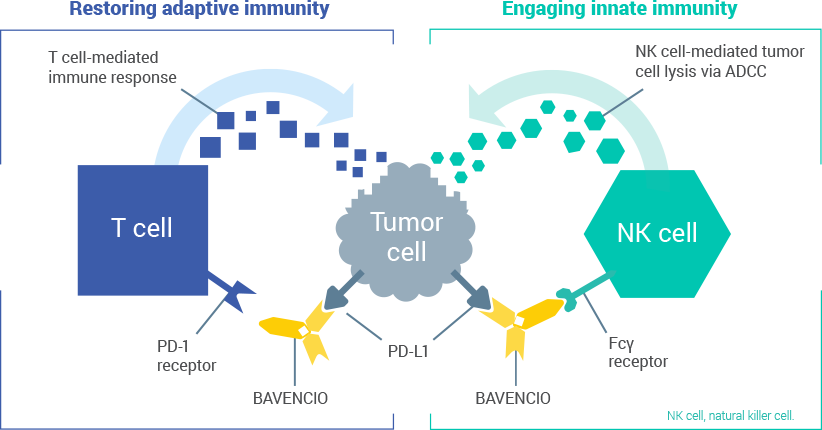
Mechanism Of Action Bavencio Sup Sup Avelumab Hcp Safety Info

Metastatic Mcc Bavencio Avelumab Safety Info
Komentar
Posting Komentar