Keytruda Approval For Merkel Cell Carcinoma
Merkel Cell Carcinoma. This indication is approved under accelerated approval based on tumor response rate and durability of response.

Fda Approves Keytruda For Treatment Of Merkel Cell Carcinoma Cancerconnect
This use is approved based on how many patients responded to treatment and how long they responded.

Keytruda approval for merkel cell carcinoma. This indication is approved under accelerated approval based on tumor response rate and durability of response. This indication is approved under accelerated approval based on tumor response rate and durability of response. This indication is approved under accelerated approval based on tumor response rate and durability of response.
FDA Approves Keytruda pembrolizumab for the Treatment of Patients with Recurrent Locally Advanced or Metastatic Merkel Cell Carcinoma - December 19 2018 FDA Approves Keytruda pembrolizumab for the Treatment of Patients with Hepatocellular Carcinoma HCC Who Have Been Previously Treated with Sorafenib - November 9 2018. KEYTRUDA is a prescription medicine used to treat a kind of skin cancer called Merkel cell carcinoma MCC in adults and children. This decision from the agency falls under accelerated approval.
36 rows Approval FDA Approves Mercks Keytruda pembrolizumab for Patients with. According to the National Cancer Institute approximately 1600 people in the United States are diagnosed with MCC every year. Food and Drug Administration FDA has approved KEYTRUDA Mercks anti-PD-1 therapy for the treatment of adult and pediatric patients with recurrent locally advanced or metastatic Merkel cell carcinoma MCC based on the results of the Cancer.
KEYTRUDA is currently approved in the US. The December 2018 approval covers use of the drug in people with locally advanced or metastatic MCC which occurs most often in older people and those with weakened. Food and Drug Administration FDA granted accelerated approval to the anti-PD-1 therapy Keytruda pembrolizumab for the treatment of adult and pediatric patients with Merkel cell carcinoma MCC.
The Food and Drug Administration FDA granted accelerated approval to Keytruda pembrolizumab for the treatment of adult and pediatric patients with recurrent locally advanced or metastatic Merkel cell carcinoma according to the agency. KEYTRUDA is indicated for the treatment of adult and pediatric patients with recurrent locally advanced or metastatic Merkel cell carcinoma MCC. Merkel Cell Carcinoma.
KEYTRUDA is indicated for the treatment of adult and pediatric patients with recurrent locally advanced or metastatic Merkel cell carcinoma MCC. Advanced Merkel Cell Carcinoma MCC Advanced Merkel Cell Carcinoma KEYTRUDA is indicated for the treatment of adult and pediatric patients with recurrent locally advanced or metastatic Merkel cell carcinoma MCC. This indication is approved under accelerated approval based on tumor response rate and durability of response.
On December 19 2018 Merck NYSEMRK known as MSD outside the United States and Canada announced that the US. Inc Whitehouse Station NJ for adult and pediatric patients with recurrent locally advanced or metastatic Merkel cell carcinoma MCC. KEYTRUDA may be used when your cancer has spread or returned.
According to a story from BioSpace the biopharmaceutical company Merck recently announced that the US Food and Drug Administration FDA has approved the companys cancer drug KEYTRUDA in a new indication as a therapy for metastatic or locally advanced Merkel cell carcinoma which is a rare form of skin cancer that can be challenging to treat effectively. On December 19 2018 the Food and Drug Administration granted accelerated approval to pembrolizumab KEYTRUDA Merck Co. Merkel Cell Carcinoma KEYTRUDA is indicated for the treatment of adult and pediatric patients with recurrent locally advanced or metastatic Merkel cell carcinoma MCC.
On December 19 2018 the Food and Drug Administration granted accelerated approval to pembrolizumab KEYTRUDA Merck Co. FDA Approves Keytruda for Merkel Cell Carcinoma. KEYTRUDA is indicated for the remedy of grownup and pediatric sufferers with recurrent regionally superior or metastatic Merkel cell carcinoma MCC.
This indication is accepted underneath accelerated approval primarily based on tumor response fee and sturdiness of response. Merkel Cell Carcinoma. Merkel Cell Carcinoma KEYTRUDA is indicated for the treatment of adult and pediatric patients with recurrent locally advanced or metastatic Merkel.
This use is approved based on how many patients responded to treatment and how long they responded. Merkel Cell Carcinoma KEYTRUDA is indicated for the treatment of adult and pediatric patients with recurrent locally advanced or metastatic Merkel cell carcinoma MCC. KEYTRUDA is a prescription medicine used to treat a kind of skin cancer called Merkel cell carcinoma MCC in adults and children.
KEYTRUDA may be used when your cancer has spread or returned. The Food and Drug Administration FDA has approved the immunotherapy drug pembrolizumab Keytruda to treat people with advanced Merkel cell carcinoma MCC a rare and deadly form of skin cancer.

Merkel Cell Carcinoma A Description Of 11 Cases Actas Dermo Sifiliograficas
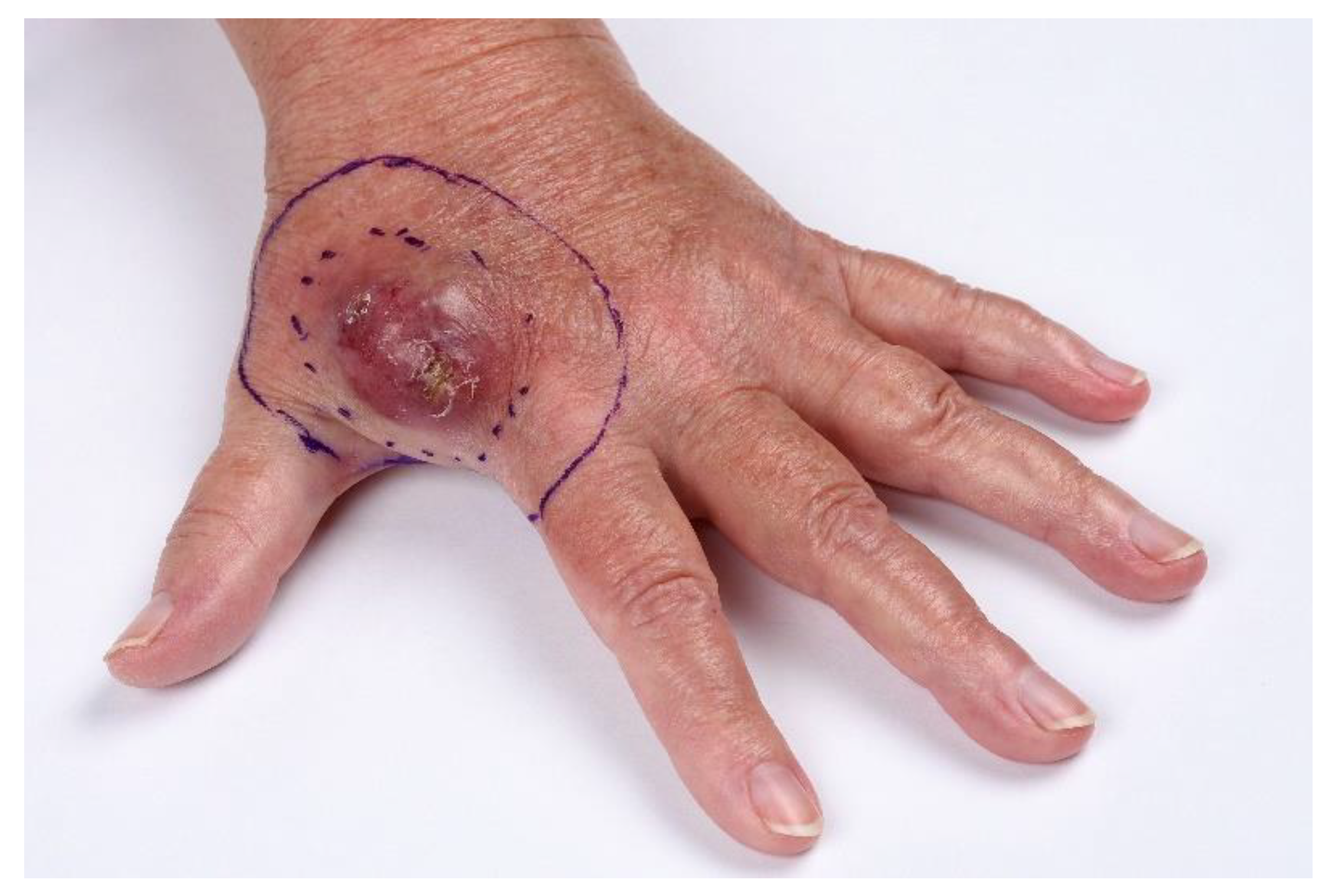
Cancers Free Full Text Management Recommendations For Merkel Cell Carcinoma A Danish Perspective Html
Komentar
Posting Komentar